ProVaHealth
The key aspect of Living Labs is the co-creation and experimental testing of products in a real-life context, providing opportunity for client validation, hands-on feedback and customer input for product development. In this way, Living Labs support companies to rapidly commercialise and scale up their innovations and products to the global markets.
In the Baltic Sea region, Living Labs exist within several health and well-being areas. The majority of Living Labs in the region work locally or regionally and not in cooperation with each other. However, there is a need for Living Labs to support small and medium-sized enterprises (SMEs) in scaling up their innovations and products to global markets.
This task is difficult if they work only locally or regionally. A weak transnational and trans-sectoral coordination of the innovation chain in the Baltic Sea region slows down the transfer of innovative products and services.
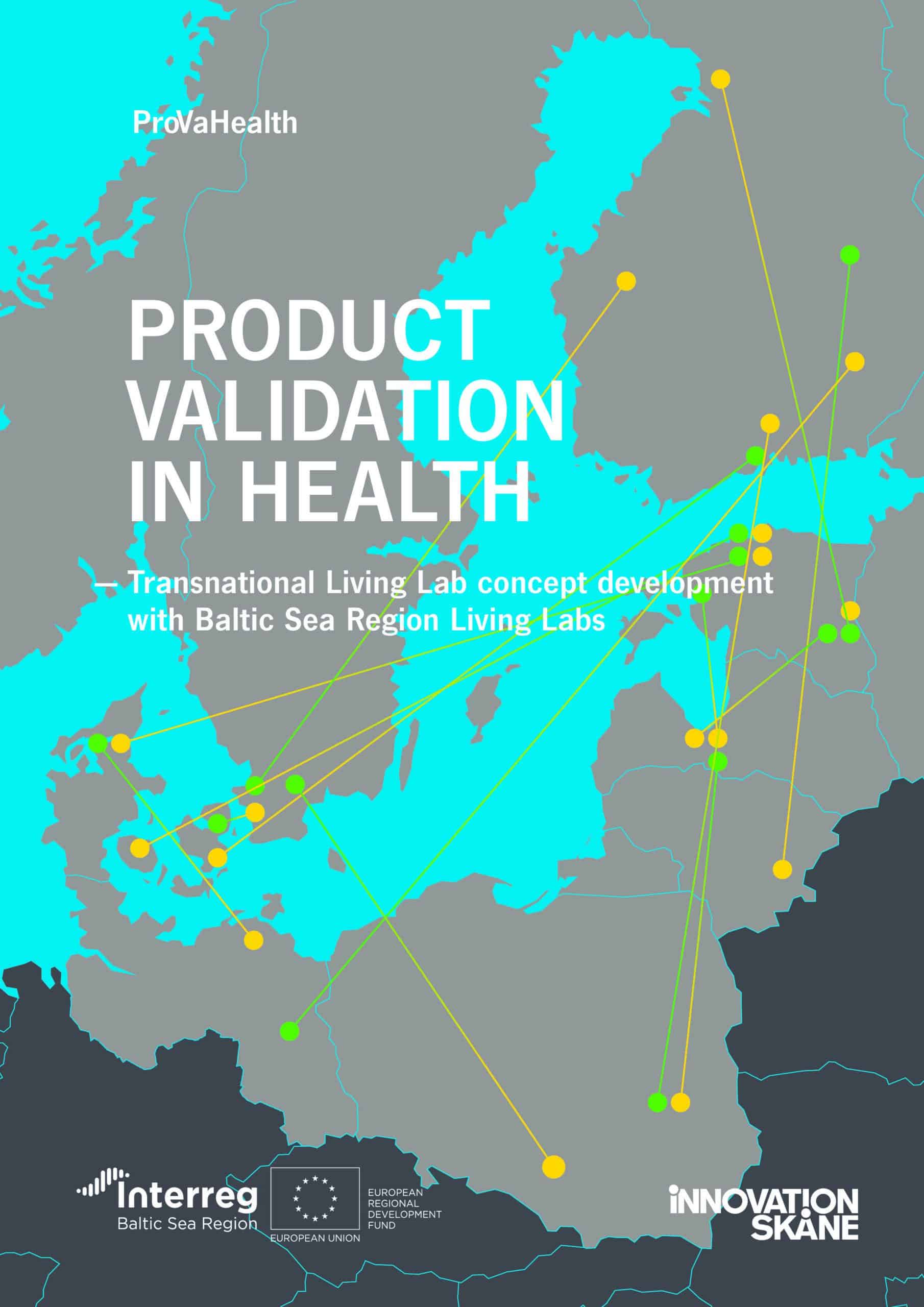
By improving the cooperation between Living Labs and SMEs, the project aimed to facilitate the validation process. Engaging 14 Labs from the Baltic Sea region, it wanted to facilitate access to Living Labs for startups and SMEs. Besides, ProVaHealth intended to tackle the challenge of a slow market uptake of innovations as well as Living Lab infrastructures serving locally or regionally only.
Besides, the development of small-scale bioenergy plants in rural areas would gear renewable energy production and sustainable development of the bionergy market in the Baltic Sea region. Facing such challenges as investment financing, suitable business models, and steady supply of biomass feedstock, the bionergy plants are experiencing tough conditions to increase their production.
Budgets
in numbers
-
2.73MillionTotal
-
2.13MillionErdf
-
0.00MillionEni + Russia
-
0.00MillionNorway
Achievements
From local to international
Thanks to the involvement to the project, the Living Labs have seen a shift of focus on the activities, from local to international. They can now offer testing and research services to international small and medium-sized enterprises (SMEs). The testing phase during the project helped them gain confidence and develop initial procedures and documents for future international testing.
Simplifications introduced
The project simplified the whole product/service validation process. Specifically, it means the easier identification of testing groups of patients, recruitment of a testing team, criteria set-up for the product to meet end-user needs and cooperation with SMEs. Besides, the project empowered the Living Labs and got them better prepared to face differences in business cultures and language issues.
New collaboration patterns established
The project established 14 collaboration pairs between one SME and one partner Living Lab. Each pair tested a specific product or service in the health sector. An example of a product validated within the project is “CoNurse” by Cognuse. It is an audio solution designed for nurses: a voice-guided tool to improve the quality of procedures and reduce medical errors and unforeseen incidents.
With EUR 2.6 million support from the European Union, the Interreg project ProVaHealth has empowered the laboratories to increase their testing offer, created a new dimension to laboratories cooperation, and contributed to an up-scale of the health innovation in the Baltic Sea region.
Outputs
Transnational Living Lab concept
Project Stories
Partners
Foundation Tallinn Science Park Tehnopol
- TownTallinn
- RegionPõhja-Eesti
- CountryEstonia
- RepresentativePiret Hirv
- Phone
- E-Mail
- Web
Haapsalu Neurological Rehabilitation Centre
- TownHaapsalu
- RegionLääne-Eesti
- CountryEstonia
- RepresentativeKadri Englas
- Phone
- E-Mail
- Web
Tallinn University
- TownTallinn
- RegionPõhja-Eesti
- CountryEstonia
- RepresentativeKatrin Niglas
- Phone
- E-Mail
- Web
North Denmark Region
- TownAalborg
- RegionNordjylland
- CountryDenmark
- RepresentativeJesper Bredmose Simonsen
- Phone
- E-Mail
- Web
Region Zealand
- TownSoroe
- RegionVest- og Sydsjælland
- CountryDenmark
- RepresentativeErik Brander
- Phone
- E-Mail
- Web
Region of Southern Denmark
- TownOdense M
- RegionFyn
- CountryDenmark
- RepresentativeJens Strandbech
- Phone
- E-Mail
- Web
South-Eastern Finland University of Applied Sciences - XAMK
- TownMikkeli
- RegionEtelä-Savo
- CountryFinland
- RepresentativeNatalia Narits
- Phone
- E-Mail
- Web
Oulu University of Applied Sciences
- TownOulu
- RegionPohjois-Pohjanmaa
- CountryFinland
- RepresentativeTiina Tervaskanto-Mäentausta
- Phone
- E-Mail
- Web
Seinäjoki University of Applied Sciences
- TownSeinäjoki
- RegionEtelä-Pohjanmaa
- CountryFinland
- RepresentativeElina Leppäkangas
- Phone
- E-Mail
- Web
Laurea University of Applied Sciences
- TownVantaa
- RegionHelsinki-Uusimaa
- CountryFinland
- RepresentativeMikko Julin
- Phone
- E-Mail
- Web
WITHDRAWAL (31.03.2019) WITENO GmbH"
- TownGreifswald
- RegionVorpommern-Greifswald
- CountryGermany
- RepresentativeAndre Huysmann
- Phone
- E-Mail
- Web
Latvian Resorts Association
- TownRiga
- RegionRīga
- CountryLatvia
- RepresentativeGunta Ušpele
- Phone
- E-Mail
- Web
Vilnius University
- TownVilnius
- RegionVilniaus apskritis
- CountryLithuania
- RepresentativeMonika Kavaliauskė
- Phone
- E-Mail
- Web
The Municipality of Lublin City
- TownLublin
- RegionLubelski
- CountryPoland
- RepresentativeMarzena Strok-Sadło
- Phone
- E-Mail
- Web
Upper Silesian Agency for Entrepreneurship and Development Ltd.
- TownGliwice
- RegionGliwicki
- CountryPoland
- RepresentativeBeata Krawczyk
- Phone
- E-Mail
- Web
Innovation Skane AB
- TownLund
- RegionSkåne län
- CountrySweden
- RepresentativeFred Kjellson
- Phone
- E-Mail
- Web
WITHDRAWAL (31.07.2019) ScanBalt
- TownFrederiksberg
- RegionByen København
- CountryDenmark
- RepresentativePeter Frank
- Phone
- E-Mail
- Web
Scanbalt
- TownTartu
- RegionLõuna-Eesti
- CountryEstonia
- RepresentativeSven Parkel
- Phone
- E-Mail
- Web
-
Project managerPiret HirvFoundation Tallinn Science Park Tehnopol
-
Legal representativeIndrek OravFoundation Tallinn Science Park Tehnopol
-
Financial managerKert KaljulaFoundation Tallinn Science Park Tehnopol
-
Communication managerKristi JõeäärFoundation Tallinn Science Park Tehnopol